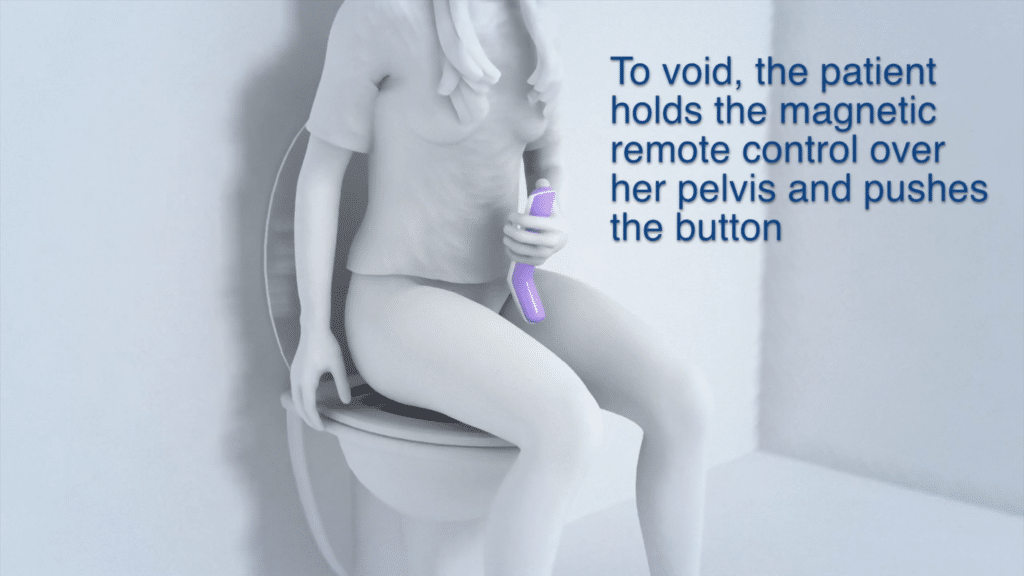
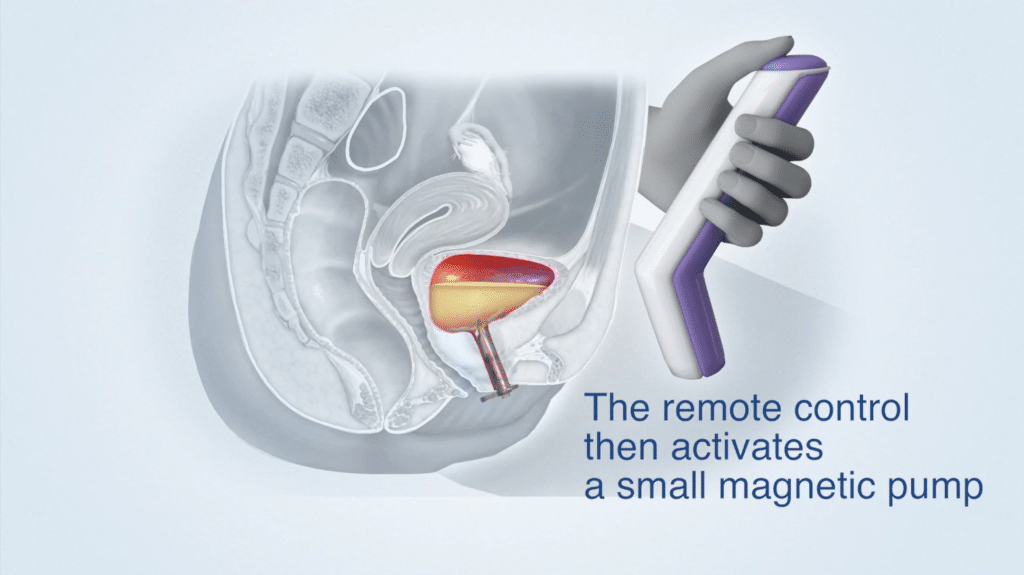
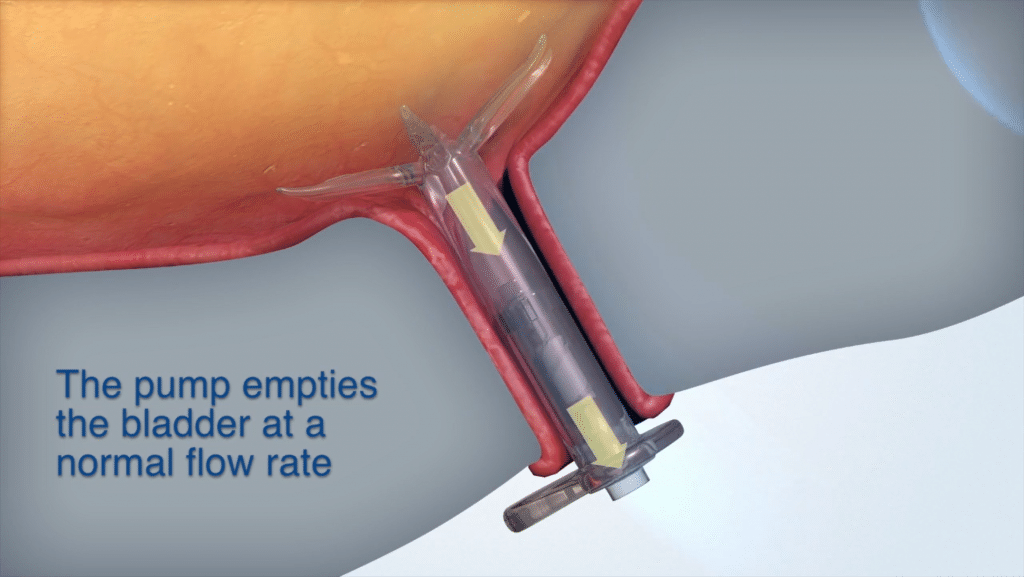
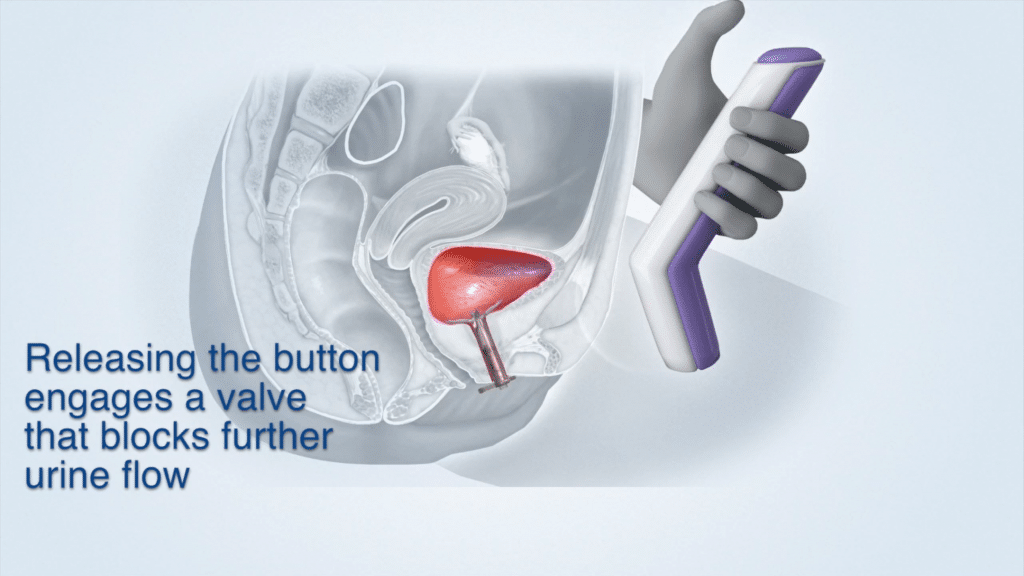
Device Use
Device Insertion and Removal
The inFlow device is available in 9 sizes in order to accommodate individual patient anatomy. Prior to initial device insertion, the inFlow Sizing device is used to measure urethral length in order to determine the appropriate inFlow device size. Urethral length measurement and initial inFlow device insertion are performed by a clinician, usually a urologist. Preparation for insertion is similar to that for a urinary catheter.
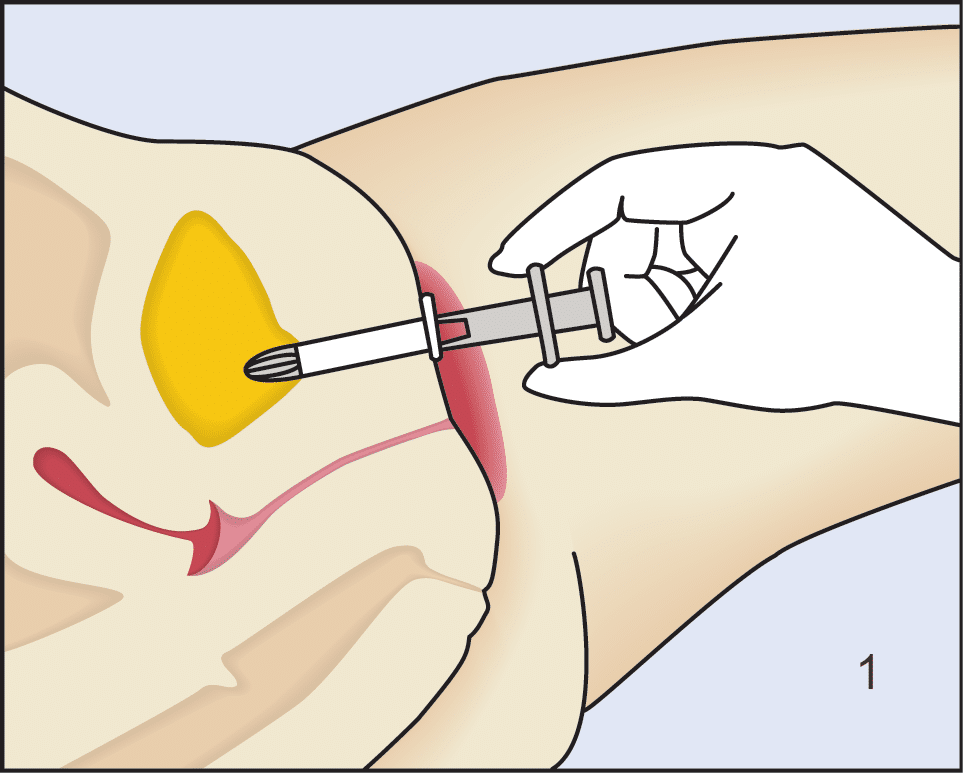
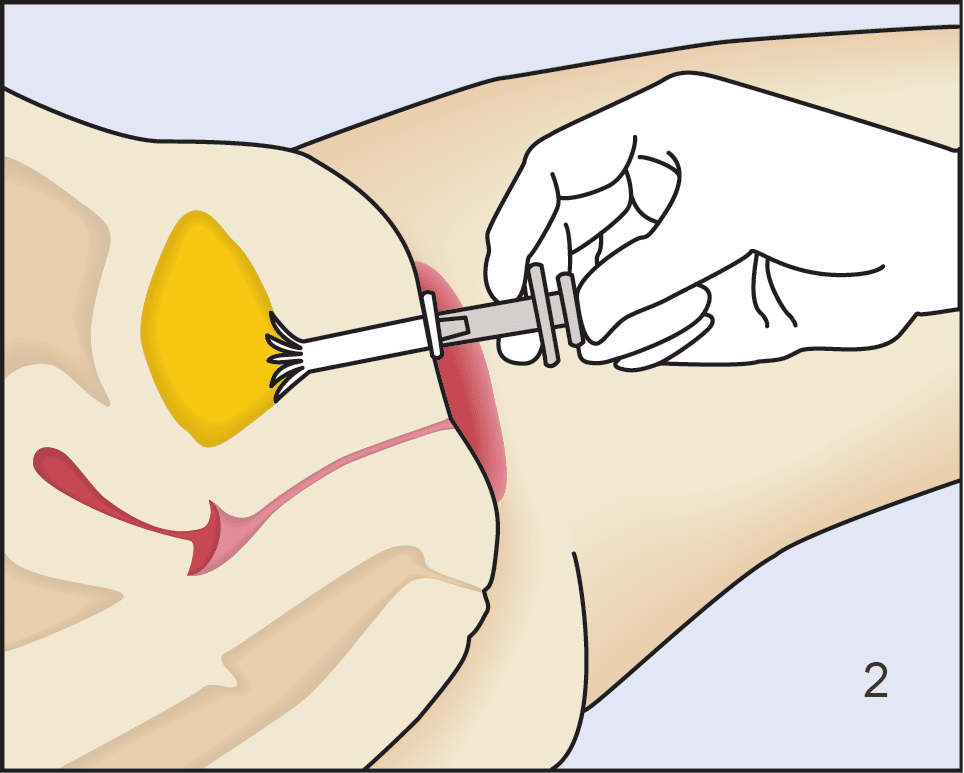
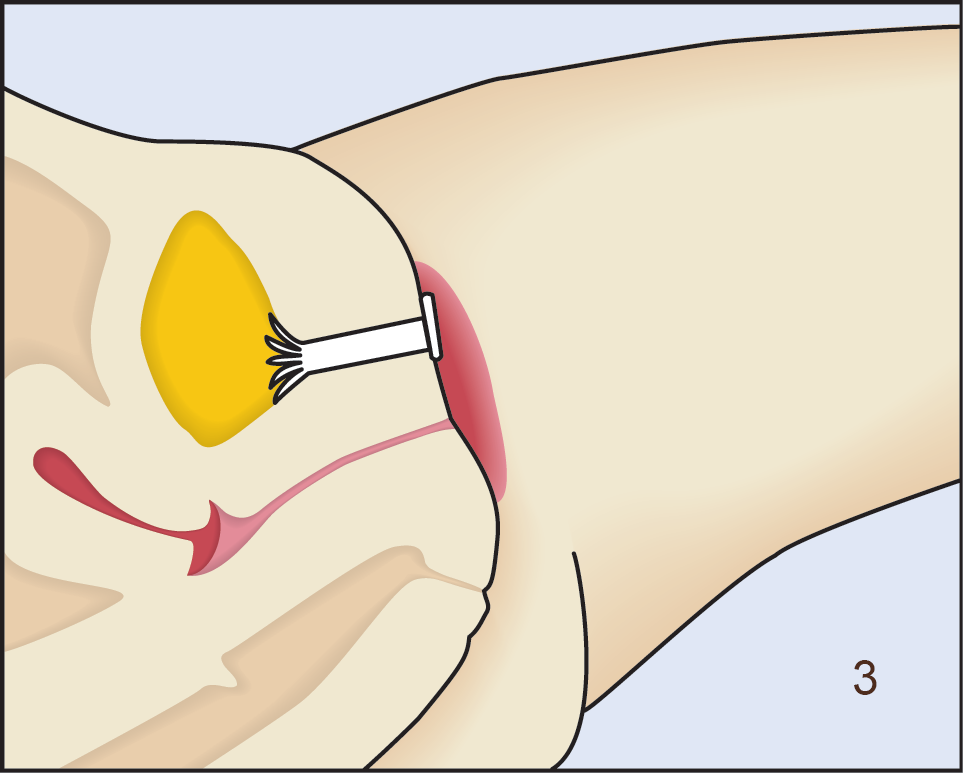
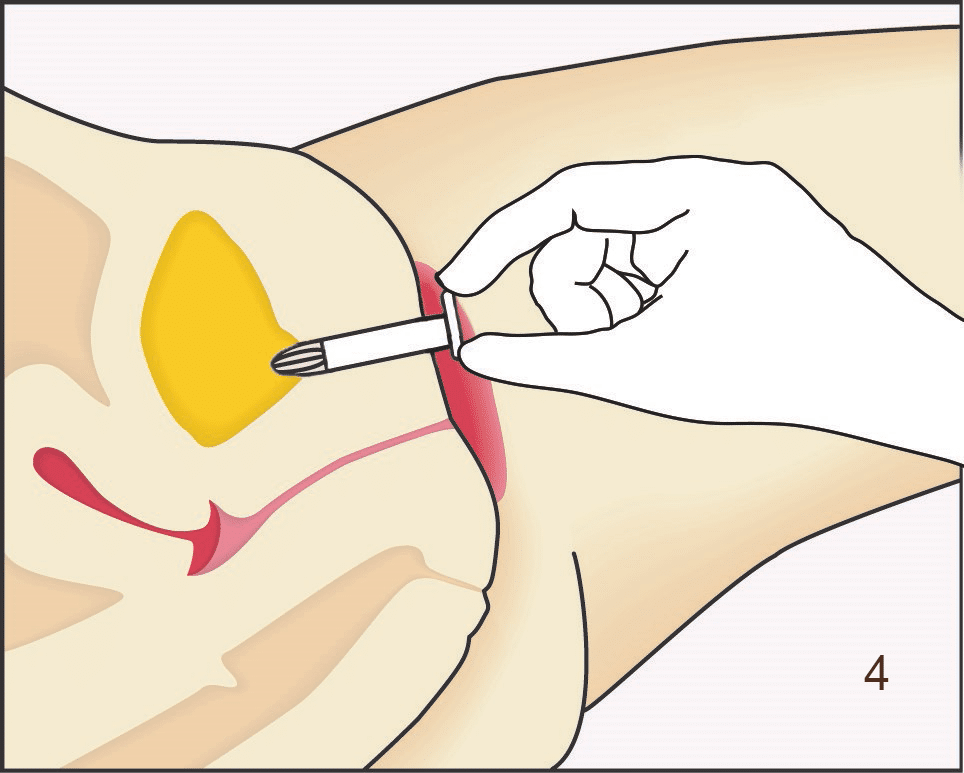
Following insertion (1), the device is deployed by pushing the plunger on its disposable introducer (2). The deployed device resides almost entirely in the urethra (3). As with an indwelling (Foley) catheter, the inFlow device should be removed and replaced every 29 days. The device can be easily and safely removed at any time, even by patients, by simply grasping its tab and pulling straight out (4).

